- 翰林提供學(xué)術(shù)活動(dòng)、國(guó)際課程、科研項(xiàng)目一站式留學(xué)背景提升服務(wù)!
- 400 888 0080
AQA A Level Chemistry復(fù)習(xí)筆記8.2.5 Vanadium Oxidation States
Vanadium Oxidation States
Vanadium Oxidation States
- Vanadium is a transition metal which has variable oxidation states
- Vanadate(V) ions can be reduced to oxidised vanadium species with the oxidation states II, III and IV by using zinc in acidic conditions
- You could be asked to carry out a test tube practical to observe the different colour changes and calculate the oxidation state of the vanadium species produced
Practical
- Add a quarter of a spatula of ammonium vanadate(V) to a test tube
- Using a dropping pipette, half-fill the test tube with 1.0 mol dm?3?hydrochloric acid and shake gently
- Note your observations
- Add one small piece of zinc, and gently shake the test tube
- Note your observations over a period of 15 minutes
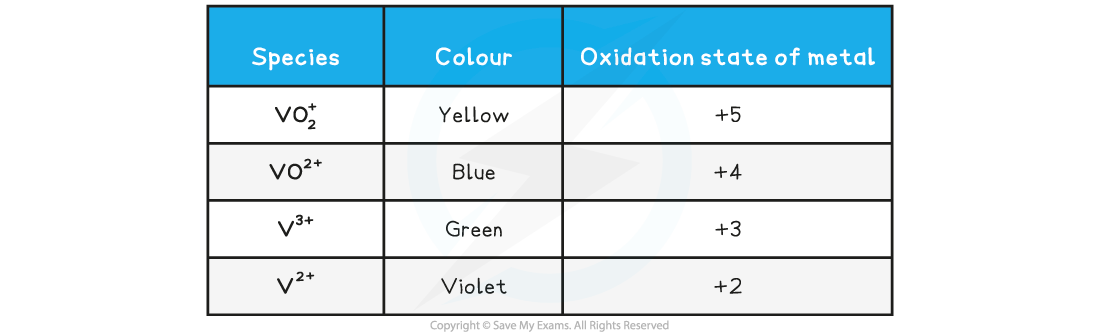
Vanadium results table
Exam Tip
V2+?is oxidised in air, therefore to observe the V2+, the test tube may need a stopper once the reduction has begun. Otherwise it turns back green because of its contact with oxygen in the air. It is oxidised back to vanadium(III).
Worked Example
What do you observe as ammonium vanadate(V) is reduced?
Answer:
The solid dissolves in the acid to form a yellow solution. This becomes a blue, then green and finally violet solution
轉(zhuǎn)載自savemyexams

最新發(fā)布
? 2025. All Rights Reserved. 滬ICP備2023009024號(hào)-1









