- 翰林提供學術活動、國際課程、科研項目一站式留學背景提升服務!
- 400 888 0080
IB DP Chemistry: HL復習筆記18.1.5 Salt Hydrolysis
Salt Hydrolysis
- An ionic salt is formed from the neutralisation reaction of an acid and base

Neutralisation?
- The ionic salt, MA, formed will dissociate in water
- Hydrolysis?is where water is used to break a bond within a compound, which results in the aqueous ions for an ionic salt
- The reaction of the salt will vary depending on the strength of the acids and bases used in the neutralisation reaction
- The use of the differing strengths of the acids and bases will directly influence the type of salt hydrolysis and the pH of the final solution
Strong Acids and Strong Bases
- A common example of this is the reaction between hydrochloric acid, HCl (aq), and sodium hydroxide (aq):
HCl (aq) + NaOH (aq) → NaCl (aq) + H2O
- The Na+?and Cl-?ions do not act as Br?nsted-Lowry?acids or bases as they can not release or accept H+?ions
- Therefore, they do not affect the pH
Strong Acid and Weak Base
- The salt formed by a strong acid such as hydrochloric acid, HCl (aq), and a weak base such as ammonia, NH3?(aq), will form an acidic solution:
HCl (aq) + NH3?(aq) → NH4Cl (aq)
- In this reaction, the conjugate acid of ammonia is formed,?NH4+, and can react with water to produce?H3O+
NH4+?(aq) + H2O (l) → H3O+?(aq) + NH3?(aq)?
- Therefore, the solution becomes more acidic
- The hydrolysis of this salt demonstrates why the equivalence point of a strong acid - weak base pH curve is below 7
Strong Base and Weak Acid
- The salt formed by a strong base such as sodium hydroxide, NaOH (aq), and a weak acid such as ethanoic acid, CH3COOH (aq), will form an acidic solution:
NaOH (aq) + CH3COOH (aq) →?CH3COONa (aq) + H2O (l)
- In this reaction, the conjugate base of ethanoic acid is produced,?CH3COO–?(aq), and this will react with water to form hydroxide ions,?OH-?(aq)
CH3COO–?(aq) + H2O (l) → CH3COOH (aq) + OH-?(aq)
- Therefore, the solution becomes more basic
- The hydrolysis of this salt demonstrates why the equivalence point of a strong base - weak acid pH curve is above 7
Weak Acid and Weak Base
- In order to determine the pH of the resulting solution of a reaction between a weak acid and weak base we must take into account the?Ka?and?Kb?values
- Using the reaction between ammonia, NH3?(aq), and ethanoic acid, CH3COOH (aq), as an example:
NH3?(aq) + CH3COOH (aq)→ CH3COONH4?(aq)
- Both the cation (positive ion) and anion?ion (negative) produced will have acid-base properties
CH3COO–?(aq) + H2O (l) → CH3COOH (aq) + OH-?(aq)
NH4+?(aq) + H2O (l) → H3O+?(aq) + NH3?(aq)?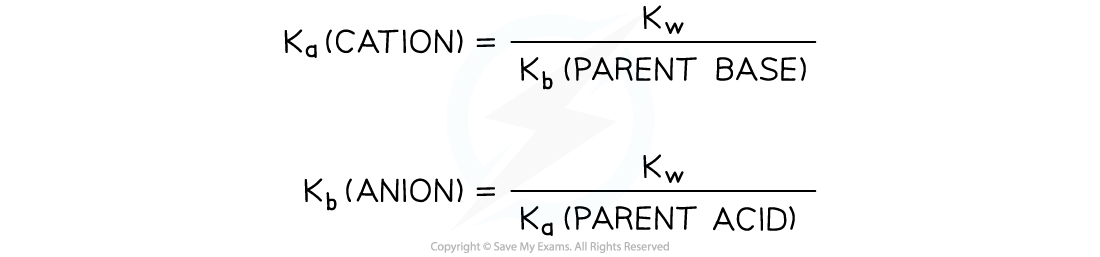
How to calculate the vales for the values of Ka?(cation) and Kb?(anion)
- If the?Ka?is larger, the solution will be acidic
- If the?Kb?is larger the solution will be basic
- If?Ka?=?Kb, then the pH will be 7
Metals
- Small metal ions that have a high charge will exhibit a high charge density
- An example is Al3+
- This makes the highly charged metal ions ideal for forming complexes as they can coordinately bond with ligands
- The complex formed can then act as a weak acid by releasing hydrogen ions when hydrolysed, H+
- The high charge density of the metal ion increases the polarity of the water molecule pulling the electrons towards itself, until the O-H bond finally breaks
[Al(H2O)6]3+?(aq) → [Al(H2O)5(OH)]2+?(aq) + H+?(aq)
- The metal ion must have a high enough charge and small radius for this to occur, consequently, 1+ and 2+ ions will not release H+ ions and therefore decrease the pH of a solution
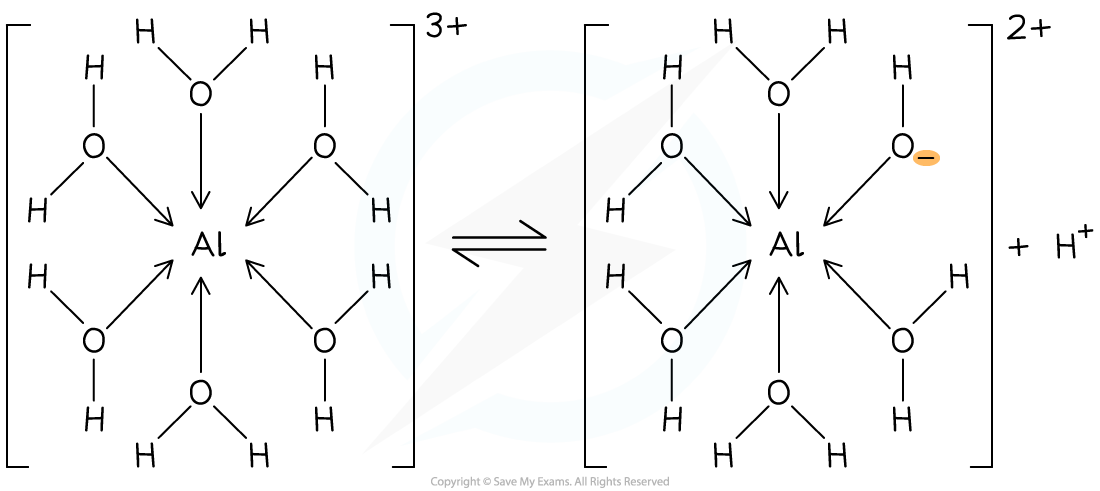
The [Al(H2O)6]3+?(aq) releases an H+ ion decreasing the pH of the solution?
轉載自savemyexams

最新發布
? 2025. All Rights Reserved. 滬ICP備2023009024號-1









