- 翰林提供學術活動、國際課程、科研項目一站式留學背景提升服務!
- 400 888 0080
CIE A Level Chemistry復習筆記1.5.3 Standard Enthalpy Change
Enthalpy Changes at Standard Conditions
- To fairly compare the changes in enthalpy between reactions, all reactions should be carried out under standard conditions
- These standard conditions are:
- A?pressure?of 101 kPa
- A?temperature?of 298 K (25?oC)
- Each substance involved in the reaction is in its normal physical state (solid, gas or liquid)
- To show that a reaction has been carried out under standard conditions, the symbol ? is used
- ΔH??= the standard enthalpy change
- These are a number of key definitions for common language relating to enthalpy change that all chemists need to know
Enthalpy definitions table

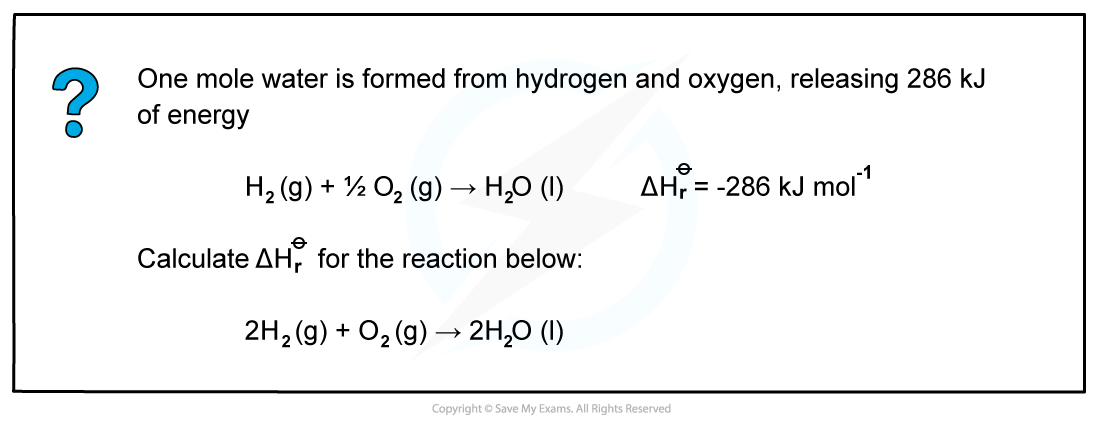
Worked example: Calculating the enthalpy change of reaction of water
Answer
Since two moles of water molecules are formed in the question above, the energy released is simply:
ΔHr??= 2 mol x (-286 kJ mol-1)= -572 kJ mol-1
Worked example: Calculating the enthalpy change of formation

Answer
Since two moles of Fe2O3?(s) are formed the total change in enthalpy for the reaction above is:
ΔHf??=? 2 x ( -824.2 kJ mol-1)= - 1648 kJ
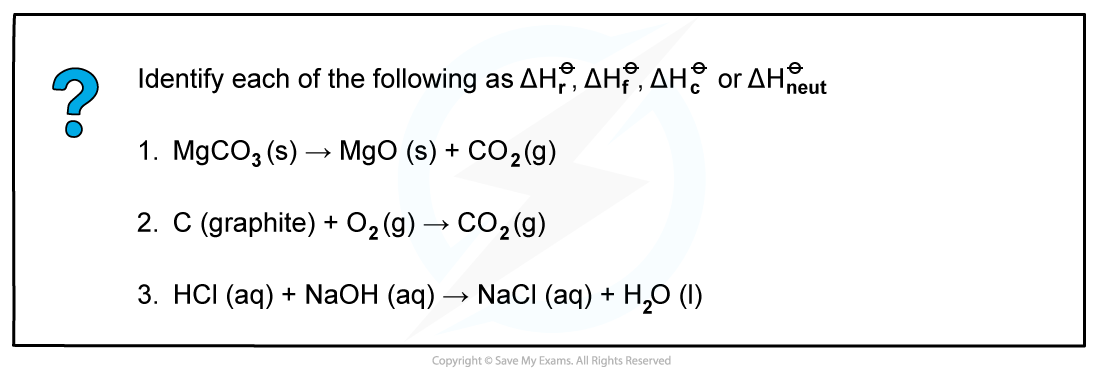
Worked example: Calculating enthalpy changes
Answer
Answer 1:?ΔHr?
Answer 2:?ΔHr??as one mole of CO2?is formed from its elements in standard state and ΔHc??as one mole of carbon is burnt in oxygen
Answer 3:?ΔHneut??as one mole of water is formed from the reaction of an acid and alkali
Exam Tip
The ΔHf??of an?element?in its standard state is zero.For example, ΔHf?of O2(g) is 0 kJ mol-1
轉載自savemyexams

最新發布
? 2025. All Rights Reserved. 滬ICP備2023009024號-1









