- 翰林提供學術活動、國際課程、科研項目一站式留學背景提升服務!
- 400 888 0080
CIE A Level Chemistry復習筆記5.4.5 Nernst Equation
The Nernst Equation
- Under non-standard conditions, the cell potential of the half-cells is shown by the symbol?Ecell
- The effect of changes in?temperature?and?ion concentration?on the?Ecell?can be deduced using the?Nernst equation
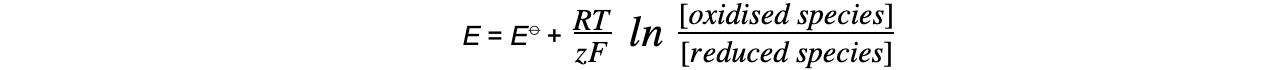
E?= electrode potential under nonstandard conditions
E??= standard electrode potential
R?= gas constant (8.31 J K-1?mol-1)
T?= temperature (kelvin, K)
z?= number of electrons transferred in the reaction
F?= Faraday constant (96 500 C mol-1)
ln = natural logarithm
- This equation can be simplified to
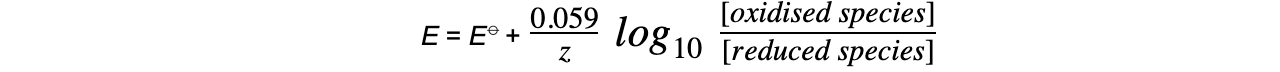
-
- At standard temperature,?R, T?and?F?are constant
- ln x = 2.303 log10?x
- The Nernst equation only depends on?aqueous ions?and?not solids or gases
- The concentrations of solids and gases are therefore set to 1.0 mol dm-3
Applying Nernst equation
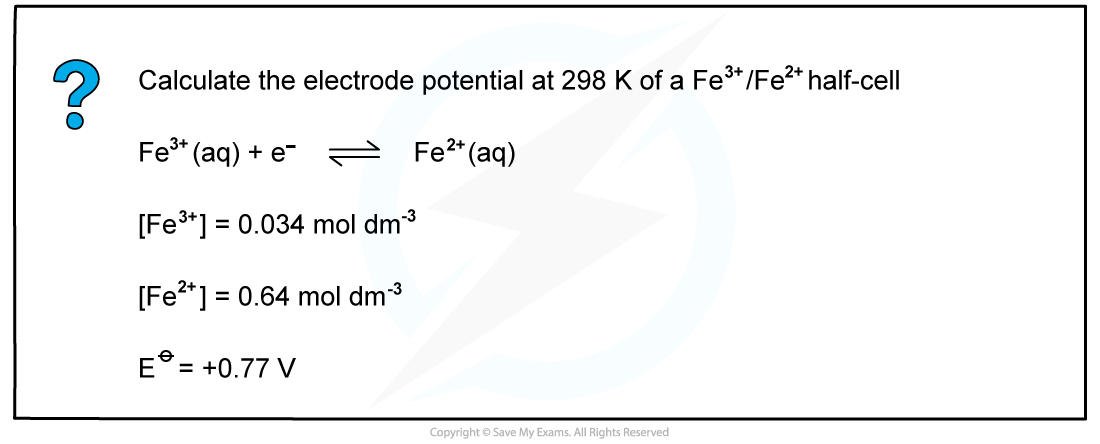
- The concentrations of ions for the Fe3+/Fe2+?half-cell are as follows:
Fe3+?(aq) + e-?? Fe2+?(aq)
[Fe3+] = 0.034 mol dm-3
[Fe2+] = 0.64 mol dm-3?
- The Nernst equation for this half-reaction is, therefore:
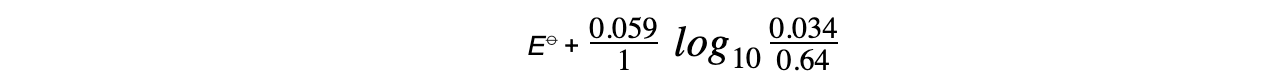
-
- The oxidised species is Fe3+?as it has a higher oxidation number (+3)
- The reduced species is Fe2+?as it has a lower oxidation number (+2)
- z is 1 as only one electron is transferred in this reaction
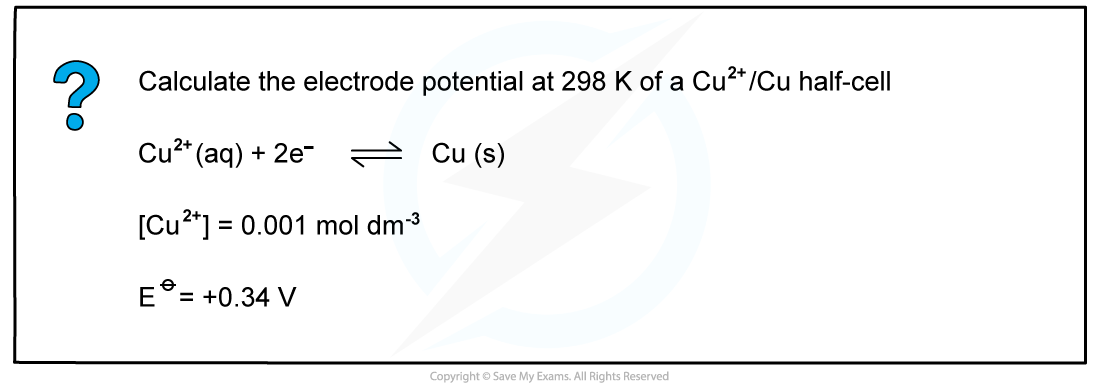
- An example of a half-cell in which?two electrons?are transferred is the Cu2+/Cu half-cell
Cu2+?(aq) + 2e-?? Cu (s)
[Cu2+] = 0.0010 mol dm-3
- The Nernst equation for this half-reaction is:
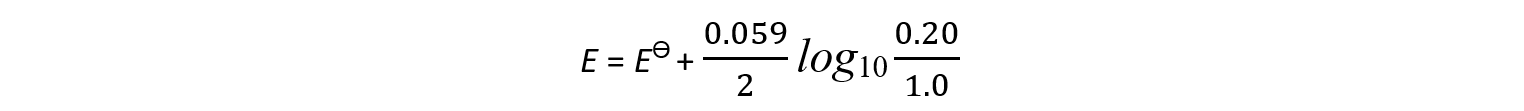
-
- The oxidised species is Cu2+?as it has a higher oxidation number (+2)
- The reduced species is Cu as it has a lower oxidation number (0)
- Cu is a solid and is not included in the Nernst equation (its concentration doesn’t change)
- z is 2 as 2 electrons are transferred in this reaction
Worked example: Calculating the electrode potential of a Fe3+/Fe2+?half-cell
Answer
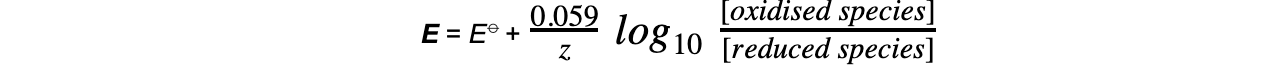
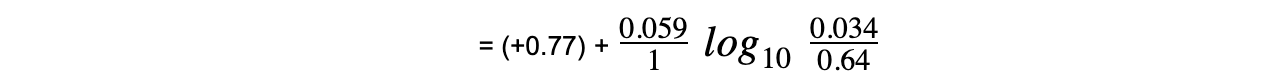
= (+0.77) + (-0.075)
= +0.69 V
Worked example: Calculating the electrode potential of a Cu2+/Cu half-cell
Answer
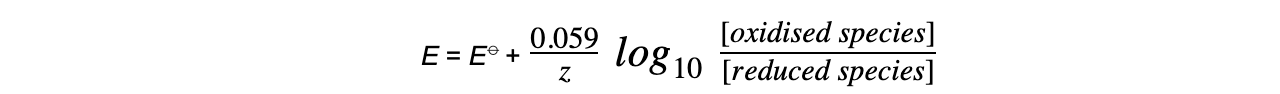
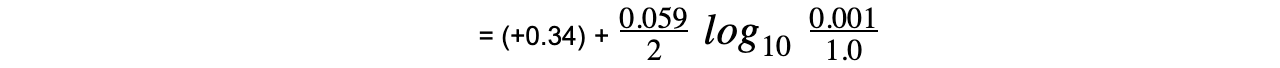
= (+0.34) + (-0.089)
= +0.25 V
Exam Tip
Make sure you always check what the temperature is. If the temperature is?not?298 K (or 25?oC) the full Nernst equation should be used.You don’t need to know how to?simplify?the Nernst equation to
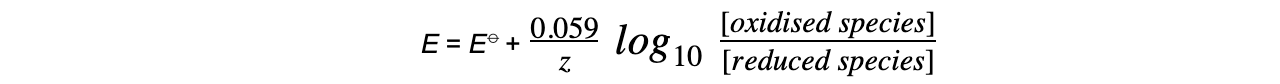
You are only expected to use the equation when the temperature is 298 K (or 25?oC).

最新發布
? 2025. All Rights Reserved. 滬ICP備2023009024號-1









