- 翰林提供學術活動、國際課程、科研項目一站式留學背景提升服務!
- 400 888 0080
CIE A Level Chemistry復習筆記5.5.5 Buffer Calculations
Calculating pH of Buffer Solutions
- The pH of a?buffer?solution?can be calculated using:
- The?Ka?of the?weak acid
- The?equilibrium concentration?of the?weak acid?and its?conjugate base?(salt)
- To determine the pH, the concentration of?hydrogen?ions?is needed which can be found using the equilibrium expression

- To simplify the calculations,?logarithms?are used such that the expression becomes:
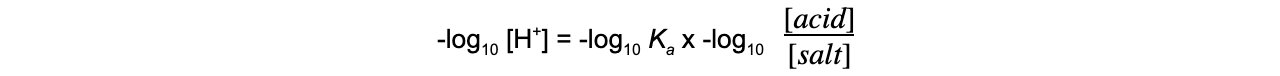
- Since -log10?[H+] = pH, the expression can also be rewritten as:
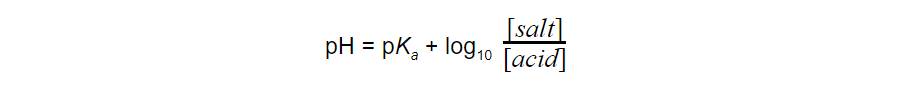
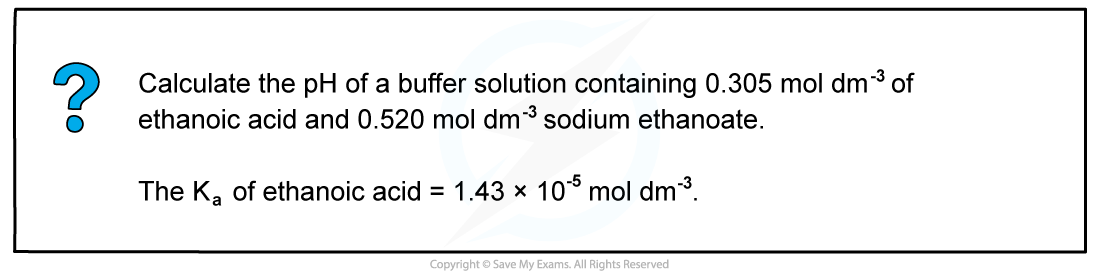
Worked Example: Calculating the pH of a buffer solution
Answer
Ethanoic acid is a weak acid that ionises as follows:
CH3COOH (aq) ? H+?(aq) + CH3COO-?(aq)
- Step 1:?Write down the equilibrium expression to find?Ka
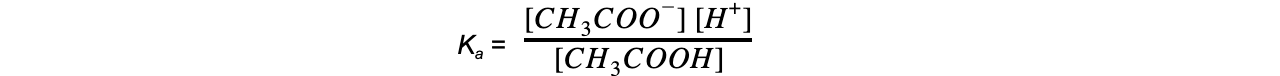
- Step 2:?Rearrange the equation to find [H+]
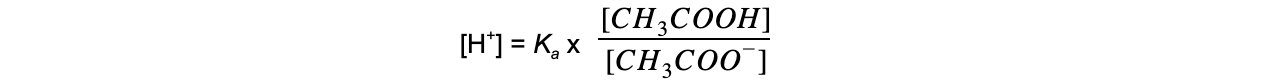
- Step 3:?Substitute the values into the expression
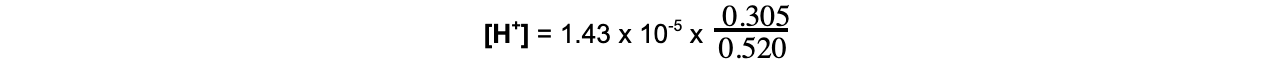
= 8.39 x 10-6?mol dm-3
- Step 4:?Calculate the pH
pH = - log [H+]
= -log 8.39 x 10-6
= 5.08
轉載自savemyexams

最新發布
? 2025. All Rights Reserved. 滬ICP備2023009024號-1









